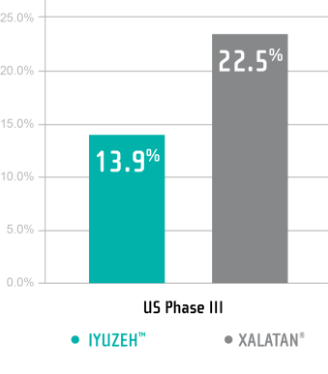
IYUZEH™ has demonstrated tolerability

Learn about treatment emergent adverse events (TEAEs).
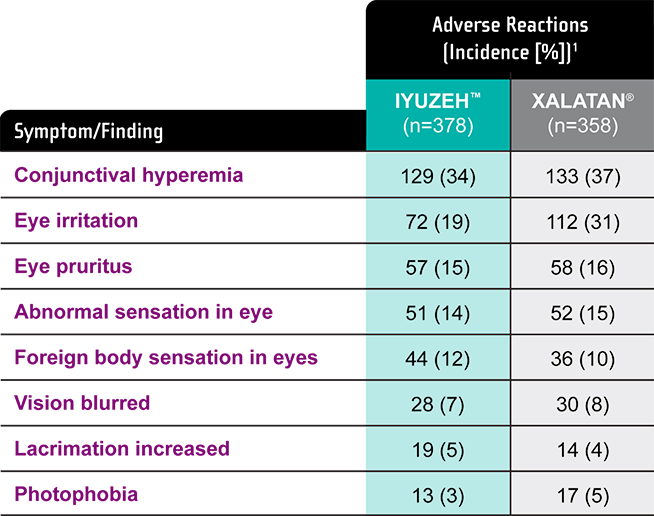
Incidence of ocular adverse events (AEs)
Treatment Emergent Adverse Events (TEAEs) in the US Phase III Trial
Hear what your colleagues are saying about IYUZEH
Challenges of any preserved ophthalmic formulation include tolerability, worsening of ocular surface disease, and that can result in compliance issues. And so anything we can do to afford a patient the ability to stay on the drug is critical in the long-term strategic success in keeping people’s vision.
Visit our Video Library to Discover Why Eye Care Professionals are Choosing IYUZEH

Things your patients may not say, but could be thinking


Ivan,† Tired of Getting a Different Generic with Every Refill
Every time Ivan picks up his generic IOP-lowering medication, it is from a different generic manufacturer. Ivan is looking for a branded medication that gives him a consistent product and experience each refill.
†Patient portrayal. Not an actual patient.
INDICATIONS AND USAGE
IYUZEH™ (latanoprost ophthalmic solution) 0.005% is a prostaglandin F2α analogue indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Known hypersensitivity to latanoprost or any other ingredients in this product.
WARNINGS AND PRECAUTIONS
IYUZEH may cause changes to pigmented tissues. Most frequently reported changes are increased pigmentation of the iris, periorbital tissue (eyelid), and eyelashes. Pigmentation is expected to increase as long as IYUZEH is administered. Iris pigmentation is likely to be permanent. Eyelid skin darkening and eyelash changes may be reversible.
IYUZEH may cause gradual change to eyelashes including increased length, thickness, and number of lashes. These changes are usually reversible upon discontinuation of treatment.
IYUZEH should be used with caution in patients with a history of intraocular inflammation (iritis/uveitis) and should generally not be used in patients with active intraocular inflammation.
IYUZEH should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Reactivation of herpes simplex keratitis has been reported during treatment with latanoprost. IYUZEH should be used with caution in patients with a history of herpetic keratitis.
Contact lenses should be removed prior to the administration of IYUZEH and may be reinserted 15 minutes after administration.
ADVERSE REACTIONS
The most common adverse reactions (5% to 35%) for IYUZEH are: conjunctival hyperemia, eye irritation, eye pruritus, abnormal sensation in eye, foreign body sensation in eyes, vision blurred, and lacrimation increased.
DRUG INTERACTIONS
The combined use of two or more prostaglandins or prostaglandin analogs including IYUZEH is not recommended. It has been shown that administration of these prostaglandin drug products more than once daily may decrease the IOP lowering effect or cause paradoxical elevations in IOP.
Please click here for the full Prescribing Information.
References: 1. IYUZEH™ (latanoprost ophthalmic solution) 0.005%. Prescribing information. Thea Pharma Inc; 2024. 2. Thea data on file. 2023. Clinical overview of T2345. 3. Bacharach J, Ahmed IIK, Sharpe ED, Korenfeld MS, Zhang S, Baudouin C. Preservative-free versus benzalkonium chloride–preserved latanoprost ophthalmic solution in patients with primary open-angle glaucoma or ocular hypertension: a phase 3 US clinical trial. Clin Ophthalmol. 2023;17:2575-2588.