Patients were satisfied or very satisfied with preservative-free latanoprost
In a real-world study* of 1872 patients who switched to preservative-free latanoprost ophthalmic solution 0.005% formulation and were treated with it for at least 3 months or were treatment-naive at the time of the study—
Those who were satisfied with their treatment included1,2:



Review factors that impacted a Preservative-Free Switch
The proportion of patients satisfied with preservative-free latanoprost ophthalmic solution 0.005% was similar among patients who had previously received preserved medication 95.3% (p<0.0001) and among those who were treatment-naive (97.3%).1
*Based on results from a multicenter, international, transverse, epidemiological survey conducted in routine private ophthalmology practices to assess level of satisfaction among preservative-free latanoprost-treated patients.1
Patients reported improved Satisfaction and Tolerability after switching to preservative-free latanoprost
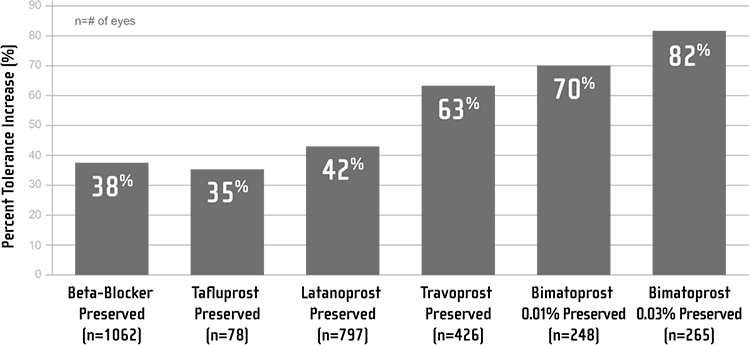
Why switch to preservative-free latanoprost?
Patients’ evaluation of their tolerance to glaucoma treatment is key to assessing treatment efficacy1
There are many factors that can make an impact on patients who switch to preservative-free latanoprost ophthalmic solution 0.005%. The most common reason for switching to preservative-free latanoprost was local intolerance or lack of efficacy, according to 1872 patients surveyed during a routine clinical examination with their ophthalmologist.1
This real-world study of patients who switched to preservative-free latanoprost ophthalmic solution 0.005% formulation and were treated with it for at least 3 months at time of study found that patient satisfaction was significantly associated with1:

Improved tolerance (better, much better, or the same as their previous treatment) (p<0.0001)

Reduction of tear substitute use (p<0.0001)

High tolerability, for those treated with preservative-free latanoprost (p<0.0001)

Absence of ocular symptoms (p<0.0004)

Perceived ease of use (as easy to use, easier to use, or much easier to use) (p<0.0001)
Hear what your colleagues are saying about IYUZEH
Having the opportunity for IYUZEH and a preservative-free latanoprost for my patients is a game changer. This will afford my patients a more well-tolerated, more comfortable, and very effective treatment approach to their glaucoma disease. So, it’s gonna be great for my patients and my practice.
Visit our Video Library to Discover Why Eye Care Professionals are Choosing IYUZEH

Things your patients may not say, but could be thinking


Isaac,† searching for satisfaction
Isaac has been using eye drops for a long time. He has not been satisfied with his current IOP-lowering drops. Then he found out about IYUZEH™ (latanoprost ophthalmic solution) 0.005%, the first and only preservative-free latanoprost, and asked his eye care professional.
†Patient portrayal. Not an actual patient.
INDICATIONS AND USAGE
IYUZEH™ (latanoprost ophthalmic solution) 0.005% is a prostaglandin F2α analogue indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Known hypersensitivity to latanoprost or any other ingredients in this product.
WARNINGS AND PRECAUTIONS
IYUZEH may cause changes to pigmented tissues. Most frequently reported changes are increased pigmentation of the iris, periorbital tissue (eyelid), and eyelashes. Pigmentation is expected to increase as long as IYUZEH is administered. Iris pigmentation is likely to be permanent. Eyelid skin darkening and eyelash changes may be reversible.
IYUZEH may cause gradual change to eyelashes including increased length, thickness, and number of lashes. These changes are usually reversible upon discontinuation of treatment.
IYUZEH should be used with caution in patients with a history of intraocular inflammation (iritis/uveitis) and should generally not be used in patients with active intraocular inflammation.
IYUZEH should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Reactivation of herpes simplex keratitis has been reported during treatment with latanoprost. IYUZEH should be used with caution in patients with a history of herpetic keratitis.
Contact lenses should be removed prior to the administration of IYUZEH and may be reinserted 15 minutes after administration.
ADVERSE REACTIONS
The most common adverse reactions (5% to 35%) for IYUZEH are: conjunctival hyperemia, eye irritation, eye pruritus, abnormal sensation in eye, foreign body sensation in eyes, vision blurred, and lacrimation increased.
DRUG INTERACTIONS
The combined use of two or more prostaglandins or prostaglandin analogs including IYUZEH is not recommended. It has been shown that administration of these prostaglandin drug products more than once daily may decrease the IOP lowering effect or cause paradoxical elevations in IOP.
Please click here for the full Prescribing Information.
References: 1. Erb C, Stalmans I, Iliev M, Muñoz-Negrete FJ. Real-world study on patient satisfaction and tolerability after switching to preservative-free latanoprost. Clin Ophthalmol. 2021;15:931-938. 2. Thea data on file. 2022. Monoprost discussion guide.