Efficacy Results from IYUZEH™

An eye care professional shares his perspective on IYUZEH™.
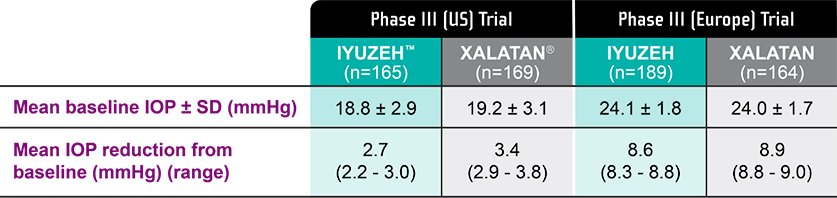
IOP-lowering efficacy from latanoprost, without preservatives
*In the US clinical trial, the mean IOP baseline was 18.8 mmHg for IYUZEH (n=165) and 19.2 mmHg for XALATAN® (n=169) compared with 24.1 mmHg for IYUZEH (n=189) and 24 mmHg for XALATAN (n=164) in the European clinical trial, accounting for the smaller yet still clinically meaningful IOP-lowering effect.1-4
XALATAN is a registered trademark of Pfizer PFE Holdings 4 LLC, a Viatris Company.
Hear what your colleagues are saying about IYUZEH
In the study, there was a very important differentiating factor that most new agents don’t have–the bar. And the bar in this study was branded XALATAN. Most of the Phase III trials for approval of medicines in the US for glaucoma and ocular hypertension, the comparator is beta blockers. And so, for IYUZEH, in its pivotal studies, to go against XALATAN, that’s a pretty high bar.
Visit our Video Library to Hear Why Eye Care Professionals are Choosing IYUZEH

Things your patients may not say, but could be thinking


Eileen,† Seeking Efficacy Without Extra Ingredients
Eileen has been on a latanoprost generic for a few years and has done her homework on the different ingredients in ophthalmic solutions that lower IOP. While she’s really interested in a treatment without benzalkonium chloride (BAK), she doesn’t want to risk any increase in IOP.
†Patient portrayal. Not an actual patient.
INDICATIONS AND USAGE
IYUZEH™ (latanoprost ophthalmic solution) 0.005% is a prostaglandin F2α analogue indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Known hypersensitivity to latanoprost or any other ingredients in this product.
WARNINGS AND PRECAUTIONS
IYUZEH may cause changes to pigmented tissues. Most frequently reported changes are increased pigmentation of the iris, periorbital tissue (eyelid), and eyelashes. Pigmentation is expected to increase as long as IYUZEH is administered. Iris pigmentation is likely to be permanent. Eyelid skin darkening and eyelash changes may be reversible.
IYUZEH may cause gradual change to eyelashes including increased length, thickness, and number of lashes. These changes are usually reversible upon discontinuation of treatment.
IYUZEH should be used with caution in patients with a history of intraocular inflammation (iritis/uveitis) and should generally not be used in patients with active intraocular inflammation.
IYUZEH should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Reactivation of herpes simplex keratitis has been reported during treatment with latanoprost. IYUZEH should be used with caution in patients with a history of herpetic keratitis.
Contact lenses should be removed prior to the administration of IYUZEH and may be reinserted 15 minutes after administration.
ADVERSE REACTIONS
The most common adverse reactions (5% to 35%) for IYUZEH are: conjunctival hyperemia, eye irritation, eye pruritus, abnormal sensation in eye, foreign body sensation in eyes, vision blurred, and lacrimation increased.
DRUG INTERACTIONS
The combined use of two or more prostaglandins or prostaglandin analogs including IYUZEH is not recommended. It has been shown that administration of these prostaglandin drug products more than once daily may decrease the IOP lowering effect or cause paradoxical elevations in IOP.
Please click here for the full Prescribing Information.
References: 1. IYUZEH™ (latanoprost ophthalmic solution) 0.005%. Prescribing information. Thea Pharma Inc; 2024. 2. Bacharach J, Ahmed IIK, Sharpe ED, Korenfeld MS, Zhang S, Baudouin C. Preservative-free versus benzalkonium chloride–preserved latanoprost ophthalmic solution in patients with primary open-angle glaucoma or ocular hypertension: a phase 3 US clinical trial. Paper presented at: ASCRS, ASOA Symposium & Congress; May 5-8, 2023; San Diego, CA. 3. Thea data on file. 2023. Clinical overview of T2345. 4. Rouland JF, Traverso CE, Stalmans I, et al. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol. 2012;97(2):196-200. 5. Thea data on file. 2023. IYUZEH clinical data deep dive.